”"Expanded carrier screening [is an] acceptable strateg[y] for prepregnancy and prenatal carrier screening" -- ACOG Committee Opinion 690
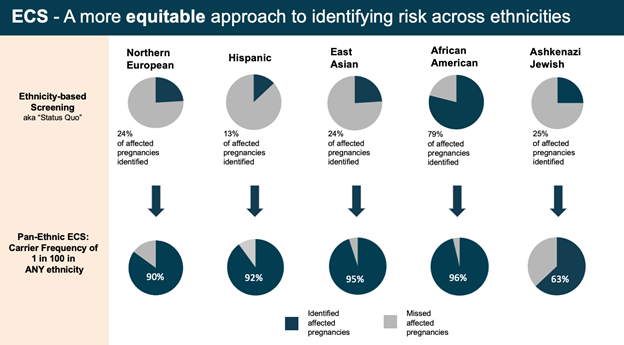
”"Relative to pan-ethnic ECS, ethnicity-based guidelines would have identified only 23% of carriers in the study cohort, with large differences per ethnicity." -- Kaseniit et al, 2020
Payers Consideration of ECS
Carrier screening has been a core component of fetal risk assessment for decades; however, an ethnicity-guided approach leads to inequitable care as our population diversifies and our knowledge of genetics expands. ECS is a modernized approach that enables risk assessment for serious genetic disorders regardless of ethnicity, and allows patients to make informed pregnancy decisions in alignment with their personal beliefs and values. Identifying potential diseases earlier can significantly reduce costs associated with treatment and care.
- Equitable Carrier Screening
- Patients Utilize ECS Results to Inform Reproductive Decisions
- Professional Societies Recognize the Benefits
- Parameters to ECS are in Place
ECS is Proven to be Actionable.
A survey of at-risk couples found that 77% of preconception couples alter reproductive management including pursuing:
- IVF
- Adoption
- Embryo donation
The same survey of at-risk couples found that 37% of those screened during pregnancy elected prenatal diagnostic testing.
Knowing early allows families to make the diagnostic testing decision that is right for them.
The Following Professional Societies Recognize the Benefits of ECS
- The American College of Obstetrics and Gynecology (ACOG)
- The American College of Medical Genetics and Genomics (ACMG)
- The Society for Maternal Fetal Medicine (SMFM)
- The National Society of Genetic Counselors (NSGC)
- The Perinatal Quality Foundation (PDQ)
- The European Society of Human Genetics (ESHG)
- The National Association of Nurse Practitioners in Women’s Health (NPWH)
Medical Societies Have Criteria and Guardrails for ECS Testing
ACOG Criteria for Disease Inclusion
- Carrier frequency ≤ 1 in 100, and/or
- Have a well-defined phenotype, and/or
- Have a detrimental effect on quality of life, and/or
- Cause cognitive or physical impairment, and/or
- Require surgical or medical intervention, and or
- Have early-childhood onset.
ACMG Criteria for Disease Inclusion
- Disorders should be severe in nature
- Disorders should have childhood onset
- Causative genes, mutations, and carrier frequencies should be known to allow for meaningful residual risk assessment
- Must be validated clinical association between mutation and severity
- Laboratories must comply with ACMG standards for Clinical Genetic Laboratories
News For Payers
Learn how you can help make ECS accessible
Keep up to date and find ways to help